Welcome to the DHC Regulatory Roundup, a new feature courtesy of our stableful of Regulatory experts. For this quarter's inaugural edition, we've got a 2-for-1 content drop in which:
CATT is a CBER center led program enabling (very) early-stage informal pre-submission interaction with FDA regarding novel advanced manufacturing technologies. The program facilitates discussion regarding issues related to the implementation of these technologies in the development of CBER-regulated products, including cell and gene therapies. For example, novel manufacturing technology enables:
Frequently asked questions about the CATT program include:
What should I include in my request for CATT?
Refer to the information provided here. We also recommend that you request a confirmation of receipt.
How long does it generally take to get a response to the request for a meeting?
There is currently no formal timeline for CBER to respond to a meeting request. However, it appears that a response is typically issued within 6 weeks of submission of the request. Be sure to include the word “CATT” in the subject line to ensure that it is routed to the relevant personnel. It is DHC’s recent experience that follow-on email communication with CBER beginning 30-days after submission of the meeting request could be beneficial.
What are the criteria for granting a meeting?
The decision to grant a meeting depends on:
What % of requests for meetings are being granted?
About 25-30% of the submissions are granted a meeting. In the event a meeting is not granted, CBER may still elect to provide some written feedback via email.
What is the timeline for scheduling a CATT meeting?
There is currently no formal timeline for the scheduling of a meeting. The timeline is dependent on the availability of FDA subject matter experts (SMEs) capable of providing feedback and participating in a meeting. However, a meeting is typically scheduled within 4 weeks from FDA’s communication agreeing to hold a CATT meeting.
What is the format of the meeting?
There is the option for either videoconference or a face-to-face meeting. The sponsor should indicate their preference for CBER’s consideration. Once a meeting is granted, the sponsor should prepare a presentation summarizing background information and providing questions to be addressed by FDA. Please note that product-specific questions are out of scope with discussions being limited to the featured innovative technology or regulatory pathway considerations. FDA/CBER SMEs will be invited as appropriate. The sponsor should provide presentation slides to CBER ~3 days prior to the meeting. The presentation should be sufficiently short as to leave the majority of time for an interactive discussion. Currently, CBER does not provide a formal written response following the meeting but informal follow-up email communications are common (e.g., if any clarification is needed or if there are remaining unanswered questions).
The Office of Therapeutic Products (OTP) launched on February 26, 2023, as a reorganization of the former Office of Tissues and Advanced Therapies (OTAT) into a super office. The super office has 6 new offices incorporated under its organizational structure. The 6 new offices were created by raising 2 of the former Divisions to the status of Office, and splitting 2 other Divisions into 2 Offices each. The table below shows how the former Divisions were reorganized into the new Offices.

Multiple new branches were created within the recently constituted 6 offices, as shown in the organizational chart below. Some of the branches may not be launched until more of the new reviewer and branch leadership positions are filled. The structure was designed to allow room for staffing growth.
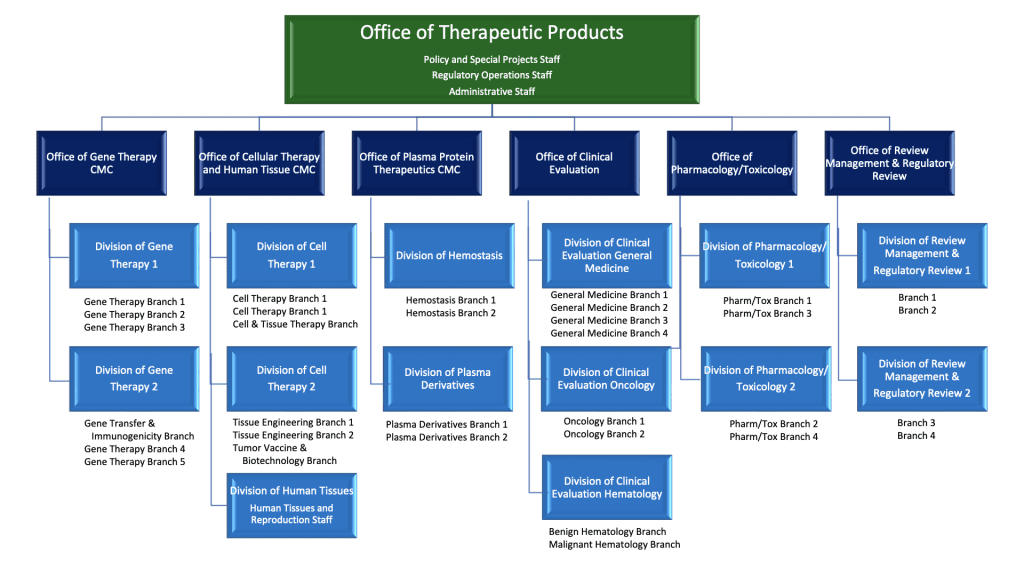
OTP has more than 100 new positions funded by the PDUFA VII reauthorization. While CBER has committed to prioritizing recruitment activities, a hiring project on this scale is unprecedented and will take months to years to complete. CBER has prioritized filling the leadership positions in OTP taking a top-down approach. Recruitment for the director of the Super Office remains ongoing. At the launch date of OTP, many familiar OTAT leaders were in acting positions at the Office and Division levels. Many are currently covering multiple positions. A list of OTP supervisors is included in the CBER Key Staff Directory. Having supervisors in place will facilitate recruitment of review staff for the numerous new vacancies.
Stakeholders have many questions about how the reorganization will affect them in the short and long term. OTP posted a Q&A page on the Establishment of the Office of Therapeutic Products. The following points from the Q&A are likely to be of broad interest: